 京公网安备11010802034965号
京ICP备13020181号-2
京公网安备11010802034965号
京ICP备13020181号-2
《中国塑料》编辑部 ©2008-2024 版权所有
地址:北京市海淀区阜成路11号 邮编:100048
编辑部:010-68985541 联系信箱:cp@plaschina.com.cn
广告部/发行部:010-68985253 本系统由北京玛格泰克科技发展有限公司设计开发

中国塑料 ›› 2023, Vol. 37 ›› Issue (1): 90-98.DOI: 10.19491/j.issn.1001-9278.2023.01.014
收稿日期:2022-10-17
出版日期:2023-01-26
发布日期:2023-01-26
通讯作者:
黄金保(1976—),男,教授,固体废弃物热化学转化利用及分子模拟,huangjinbao76@ 126.com作者简介:第一联系人:地址:北京市海淀区阜成路11号《中国塑料》杂志社广告部基金资助:
ZHOU Mei, LI Sijia, XU Weifeng, HUANG Jinbao( ), LUO Xiaosong, WU Lei
), LUO Xiaosong, WU Lei
Received:2022-10-17
Online:2023-01-26
Published:2023-01-26
Contact:
HUANG Jinbao
E-mail:huangjinbao76@ 126.com
摘要:
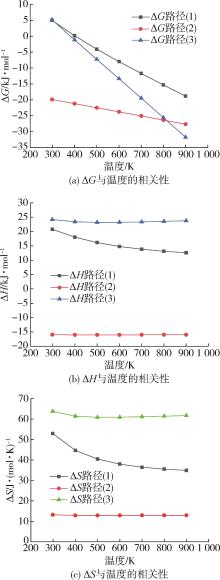
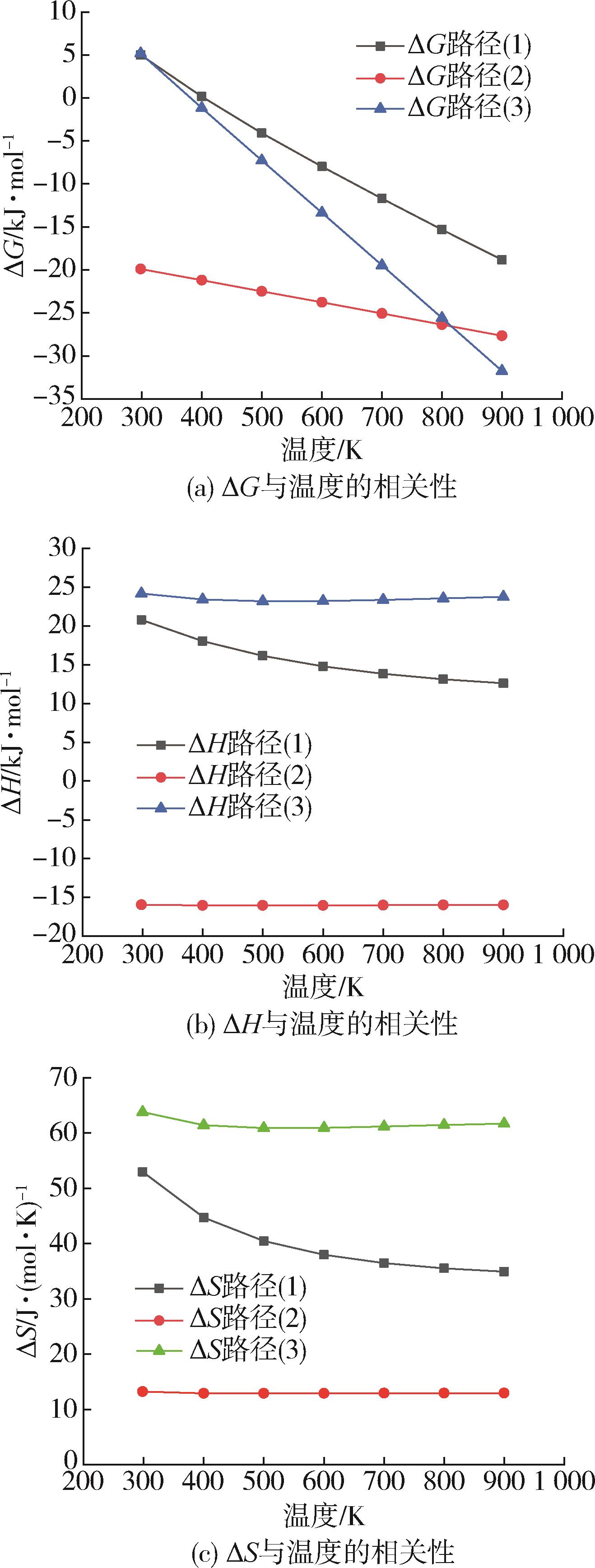
采用密度泛函理论B3P86/6⁃31++G(d,p)方法对对苯二甲酸乙二醇酯二聚体的水/醇/氨解反应机理进行了理论研究。设计了水/醇/氨降解过程的各种可能反应路径,对参与反应的各种中间体、过渡态及产物进行了几何结构优化和频率计算以获得热力学与动力学参数值,分析了对苯二甲酸乙二醇酯二聚体主链酯键中的酰氧键位置水/醇/氨降解的反应机理。结果表明,水/醇/氨解能够降低对苯二甲酸乙二醇酯二聚体主链酯键中的酰氧键裂解的反应活化能,使反应更易于进行,其中水解中主要基元反应步的反应能垒最高约为169.0 kJ/mol,氨解最低约为153.0 kJ/mol,其次是醇解约为155.0 kJ/mol。
中图分类号:
周梅, 李思佳, 徐玮峰, 黄金保, 罗小松, 吴雷. 对苯二甲酸乙二醇酯二聚体水/醇/氨解机理的理论研究[J]. 中国塑料, 2023, 37(1): 90-98.
ZHOU Mei, LI Sijia, XU Weifeng, HUANG Jinbao, LUO Xiaosong, WU Lei. Theoretical study on hydrolysis/alcoholysis/ammonolysis mechanisms of ethylene terephthalate dimer[J]. China Plastics, 2023, 37(1): 90-98.
| 温度/K | 水解/醇解/氨解反应[路径(1)/路径(2)路径(3)] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | |
| 298 | 20.8 | 5.0 | 53.0 | -15.9 | -19.9 | 13.2 | 24.2 | 5.2 | 63.8 |
| 400 | 18.1 | 0.2 | 44.7 | -16.0 | -21.2 | 12.9 | 23.4 | -1.1 | 61.4 |
| 500 | 16.2 | -4.1 | 40.5 | -16.0 | -22.5 | 12.9 | 23.2 | -7.3 | 60.9 |
| 600 | 14.8 | -8.0 | 38.0 | -16.0 | -23.8 | 12.9 | 23.2 | -13.4 | 61.0 |
| 700 | 13.9 | -11.7 | 36.5 | -16.0 | -25.1 | 12.9 | 23.4 | -19.5 | 61.2 |
| 800 | 13.1 | -15.3 | 35.6 | -16.0 | -26.4 | 13.0 | 23.6 | -25.6 | 61.5 |
| 900 | 12.6 | -18.8 | 34.9 | -16.0 | -27.7 | 13.0 | 23.8 | -31.8 | 61.7 |
| 温度/K | 水解/醇解/氨解反应[路径(1)/路径(2)路径(3)] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | ΔH/ kJ·mol-1 | ΔG/ kJ·mol-1 | ΔS/ J·(mol·K)-1 | |
| 298 | 20.8 | 5.0 | 53.0 | -15.9 | -19.9 | 13.2 | 24.2 | 5.2 | 63.8 |
| 400 | 18.1 | 0.2 | 44.7 | -16.0 | -21.2 | 12.9 | 23.4 | -1.1 | 61.4 |
| 500 | 16.2 | -4.1 | 40.5 | -16.0 | -22.5 | 12.9 | 23.2 | -7.3 | 60.9 |
| 600 | 14.8 | -8.0 | 38.0 | -16.0 | -23.8 | 12.9 | 23.2 | -13.4 | 61.0 |
| 700 | 13.9 | -11.7 | 36.5 | -16.0 | -25.1 | 12.9 | 23.4 | -19.5 | 61.2 |
| 800 | 13.1 | -15.3 | 35.6 | -16.0 | -26.4 | 13.0 | 23.6 | -25.6 | 61.5 |
| 900 | 12.6 | -18.8 | 34.9 | -16.0 | -27.7 | 13.0 | 23.8 | -31.8 | 61.7 |
| 1 | SAMAK N A, JIA Y, SHARSHAR M M, et al. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling[J]. Environment International, 2020, 145: 106144. |
| 2 | JANKAUSKAITE V. Recycled polyethylene terephthalate waste for different application solutions[J]. Environmental Research, Engineering and Management, 2016, 72(1): 5⁃7. |
| 3 | DAS S K, ESHKALAK S K, CHINNAPPAN A, et al. Plastic recycling of polyethylene terephthalate (PET) and polyhydroxybutyrate (PHB)⁃A comprehensive review[J]. Materials Circular Economy, 2021, 3(1): 1⁃22. |
| 4 | ZIMMERMANN W. Biocatalytic recycling of polyethylene terephthalate plastic[J]. Philosophical Transactions of The Royal Society A, 2020, 378(2176): 20190273. |
| 5 | WEI R, ZIMMERMANN W. Biocatalysis as a green route for recycling the recalcitrant plastic polyethylene terephthalate[J]. Microbial Biotechnology, 2017, 10(6): 1 302. |
| 6 | RAHEEM A B, NOOR Z Z, HASSAN A, et al. Current developments in chemical recycling of post⁃consumer polyethylene terephthalate wastes for new materials production: a review[J]. Journal of Cleaner Production, 2019, 225: 1 052⁃1 064. |
| 7 | AL⁃SABAGH A M, YEHIA F Z, ESHAQ G, et al. Greener routes for recycling of polyethylene terephthalate[J]. Egyptian Journal of Petroleum, 2016, 25(1): 53⁃64. |
| 8 | SOJOBI A O, NWOBODO S E, ALADEGBOYE O J. Recycling of polyethylene terephthalate (PET) plastic bottle wastes in bituminous asphaltic concrete[J]. Cogent Engineering, 2016, 3(1): 1133480. |
| 9 | KUMARTASLI S, AVINC O. Important step in sustainability: polyethylene terephthalate recycling and the recent developments[J]. Sustainability in the Textile and Apparel Industries, 2020: 1⁃19. |
| 10 | AYODEJI S O, ONI T O. Thermal pyrolysis production of liquid fuel from a mixture of polyethylene terephthalate and polystyrene[J]. Heat Transfer⁃Asian Research, 2019, 48(5): 1 648⁃1 662. |
| 11 | DHAHAK A, GRIMMER C, NEUMANN A, et al. Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques[J]. Waste Management, 2020, 106: 226⁃239. |
| 12 | HUANG W C, HUANG M S, HUANG C F, et al. Thermochemical conversion of polymer wastes into hydrocarbon fuels over various fluidizing cracking catalysts[J]. Fuel, 2010, 89(9): 2 305⁃2 316. |
| 13 | SONGIP A R, MASUDA T, KUWAHARA H, et al. Kinetic studies for catalytic cracking of heavy oil from waste plastics over REY zeolite[J]. Energy & Fuels, 1994, 8(1): 131⁃135. |
| 14 | BARTH M, OESER T, WEI R, et al. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca[J]. Biochemical Engineering Journal, 2015, 93: 222⁃228. |
| 15 | 刘 丽. 聚对苯二甲酸丁二醇酯在亚临界酸性水溶液和超/亚临界乙醇中的解聚研究[D]. 杭州: 浙江工业大学, 2011. |
| 16 | 戴娟娟. 亚临界水中聚对苯二甲酸丁二醇酯的 (催化) 解聚研究[D]. 杭州: 浙江工业大学, 2010. |
| 17 | 杨 伟. 聚酯复合材料无卤协效阻燃研究及机理的研究[D]. 合肥: 中国科学技术大学, 2012. |
| 18 | ČOLNIK M, KNEZ Ž, ŠKERGET M. Sub⁃and supercritical water for chemical recycling of polyethylene terephthalate waste[J]. Chemical Engineering Science, 2021, 233: 116389. |
| 19 | XUE Y, JOHNSTON P, BAI X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics[J]. Energy Conversion and Management, 2017, 142: 441⁃451. |
| 20 | GOLIKE R C, LASOSKI JR S W. Kinetics of hydrolysis of polyethylene terephthalate films[J]. The Journal of Physical Chemistry, 1960, 64(7): 895⁃898. |
| 21 | DU J T, SUN Q, ZENG X F, et al. ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate[J]. Chemical Engineering Science, 2020, 220: 115642. |
| 22 | CAMPANELLI J R, COOPER D G, KAMAL M R. Catalyzed hydrolysis of polyethylene terephthalate melts[J]. Journal of Applied Polymer Science, 1994, 53(8): 985⁃991. |
| 23 | YOSHIOKA T, MOTOKI T, OKUWAKI A. Kinetics of hydrolysis of poly (ethylene terephthalate) powder in sulfuric acid by a modified shrinking⁃core model[J]. Industrial & Engineering Chemistry Research, 2001, 40(1): 75⁃79. |
| 24 | 杨华光, 梁桂英, 王春晓, 等. 金属锌功能化离子液体催化PET聚酯醇解反应[J]. 材料科学与工程学报, 2012, 30(5): 747⁃751. |
| YANG H G, LIANG G Y, WANG C X, et al. PET glycolysis catalyzed by zinc task special ionic liquids[J]. Journal of Materials Science and Engineering, 2012, 30(5): 747⁃751. | |
| 25 | 姚浩余. PET 醇解协同催化体系的构建及反应机理研究[D]. 北京: 中国科学院大学 (中国科学院过程工程研究所), 2021. |
| 26 | KOJIMA H, MORI T. Gaussian 09 (Revision B. 01), 2009[J]. Chemistry letters, 2013, 42(1): 68⁃70. |
| 27 | 蒙含仙, 黄金保, 程小彩, 等. 聚对苯二甲酸乙二醇酯二聚体模化物键离能的理论研究[J]. 分子科学学报, 2021, 37(3): 261⁃267. |
| MENG H X, HUANG J B, CHENG X C, et al. Theoretical study on bond dissociation energy of polyethylene terephthalate dimer compound[J]. Journal of Molecular Science, 2021, 37(3): 261⁃267. | |
| 28 | HUANG J, MENG H, LUO X, et al. Insights into the thermal degradation mechanisms of polyethylene terephthalate dimer using DFT method[J]. Chemosphere, 2022, 291: 133112. |
| 29 | HUANG J, LI X, MENG H, et al. Studies on pyrolysis mechanisms of syndiotactic polystyrene using DFT method[J]. Chemical Physics Letters, 2020, 747: 137334. |
| 30 | 黄金保, 李新生, 潘贵英, 等. 双酚A聚碳酸酯热解机理的理论研究[J]. 工程热物理学报, 2019, 40 (8): 1 813⁃1 819. |
| HUANG J B, LI X S, PAN G Y, et al. A theoretical research on pyrolysis mechanism of poly (bisphenol A carbonate) [J]. Journal of Engineering Thermophysics, 2019, 40 (8): 1 813⁃1 819. | |
| 31 | NIU Z H, HOU W S, SHI S, et al. Study on the subcritical water hydrolysis of waste polyethylene terephthalate[J]. Fine Chemical Industry, 2015, 32(2): 126⁃132. |
| 32 | 王媚娴, 潘志彦, 戴娟娟, 等. 超/亚临界水中聚对苯二甲酸乙二醇酯的解聚[J]. 高校化学工程学报, 2011, 25(5): 904⁃910. |
| WANG M X, PAN Z Y, DAI J J, et al. Depolymerization of polyethylene terephthalate in sub⁃ and supercritical water[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(5): 904⁃910. | |
| 33 | BUXBAUM L H. The degradation of poly (ethylene terephthalate)[J]. Angewandte Chemie International Edition in English, 1968, 7(3): 182⁃190. |
| 34 | 张现刚. 超/亚临界水中聚碳酸酯/聚对苯二甲酸乙二醇酯催化解聚研究[D]. 杭州: 浙江工业大学, 2009. |
| 35 | 刘立新, 胡 达, 刘若望, 等. 聚对苯二甲酸乙二醇酯 (PET) 的解聚与反应机理研究[J]. 功能材料, 2004 (Z1): 2 576⁃2 578. |
| LIU L X, HU D, LIU R W, et al. Study on depolymerization and Reaction mechanism of polyethylene terephthalate (PET) [J]. Journal of Functional Materials, 2004 (Z1): 2 576⁃2 578. | |
| 36 | 陈 磊, 吴勇强, 倪燕慧, 等. 聚对苯二甲酸乙二醇酯在超临界甲醇中解聚的研究[J]. 高校化学工程学报, 2004, 18(5): 585⁃589. |
| CHEN L, WU Y Q, NI Y H, et al. Study on depolymerization of polyethylene terephthalate in supercritical methanol [J]. Journal of Chemical Engineering of Chinese Universities, 2004, 18(5): 585⁃589. | |
| 37 | DIMITROV N, KREHULA L K, SIROČIĆ A P, et al. Analysis of recycled PET bottles products by pyrolysis⁃gas chromatography[J]. Polymer Degradation and Stability, 2013, 98(5): 972⁃979. |
| 38 | LORENZETTI C, MANARESI P, BERTI C, et al. Chemical recovery of useful chemicals from polyester (PET) waste for resource conservation: a survey of state of the art[J]. Journal of Polymers and the Environment, 2006, 14(1): 89⁃101. |
| 39 | 俞 昊, 黄 芳, 冯淑芹, 等. 废聚对苯二甲酸乙二醇酯的高温醇解研究[J]. 合成纤维工业, 2014, 37(1): 9⁃12. |
| YU H, HUANG F, FENG S Q, et al. Study on high⁃temperature alcoholysis of waste polyethylene terephthalate [J]. Synthetic Fiber Industry, 2014, 37(1): 9⁃12. | |
| 40 | 郑 煦, 张瑞琦, 方鹏涛, 等. 离子液体催化聚对苯二甲酸乙二醇酯降解研究进展[J]. 中国科学: 化学, 2021, 51(10): 1 330⁃1 342. |
| ZHENG X, ZHANG R Q, FANG P T, et al. Research progress of ionic liquid catalyzed degradation of polyethylene terephthalate [J]. Science China: Chemistry, 2021, 51(10): 1 330⁃1 342. | |
| 41 | SHUKLA S R, HARAD A M. Aminolysis of polyethylene terephthalate waste[J]. Polymer Degradation and Stability, 2006, 91(8): 1 850⁃1 854. |
| 42 | SPYCHAJ T, FABRYCY E, KACPERSKI M. Aminolysis and aminoglycolysis of waste poly (ethylene terephthalate)[J]. Journal of Material Cycles and Waste Management, 2001, 3(1): 24⁃31. |
| 43 | YAO H, LU X, JI L, et al. Multiple hydrogen bonds promote the nonmetallic degradation process of polyethylene terephthalate with an amino acid ionic liquid catalyst[J]. Industrial & Engineering Chemistry Research, 2021, 60(10): 4 180⁃4 188. |
| 44 | HUANG J, LIU C, WU D, et al. Density functional theory studies on pyrolysis mechanism of β⁃O-4 type lignin dimer model compound[J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 98⁃108. |
| 45 | HUANG J, HE C. Pyrolysis mechanism of α⁃O-4 linkage lignin dimer: a theoretical study[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 655⁃664. |
| 46 | HUANG J, HE C, LIU C, et al. A computational study on thermal decomposition mechanism of β-1 linkage lignin dimer[J]. Computational and Theoretical Chemistry, 2015, 1054: 80⁃87. |
| [1] | 马国成, 何圳, 陈少军. 醋酸纤维素的降解性研究进展[J]. 中国塑料, 2022, 36(9): 111-121. |
| [2] | 宋进, 徐航, 邹威, 王洪, 张晨. 表面羧基化聚丙烯酸酯多孔微球的制备及其铜离子吸附性能研究[J]. 中国塑料, 2022, 36(7): 8-13. |
| [3] | 顾晓华, 吕士伟, 刘思雯, 王佳佳, 康媛媛. 废旧聚氨酯硬质泡沫塑料的降解回收及再利用[J]. 中国塑料, 2021, 35(8): 105-111. |
| [4] | 董星彤, 王向东, 孙晓红, 陈士宏. 密度泛函理论在聚合物发泡领域中的应用研究进展[J]. 中国塑料, 2021, 35(7): 126-133. |
| [5] | 毕晨曦, 李爱民. 基于乳酸水溶液循环的聚乳酸水热解聚行为研究[J]. 中国塑料, 2020, 34(8): 1-8. |
| [6] | 马巧云, 翁云宣, 张彩丽. 氯化铁催化醇解反应回收聚乳酸[J]. 中国塑料, 2020, 34(11): 73-80. |
| [7] | 顾晓华, 吕士伟, 罗鸿翔, 李燕. 催化剂对废旧PU硬泡回收再利用的影响[J]. 中国塑料, 2020, 34(10): 69-74. |
| [8] | 孟鑫 谈书航 曹齐茗 公维光 李捷 姚中阳 翟紫航 段润梓 辛忠. 基于静电纺的超疏水超亲油串晶结构聚乳酸薄膜的制备及性能研究[J]. 中国塑料, 2019, 33(4): 48-53. |
| [9] | 顾晓华, 吕士伟, 张晓华, 罗鸿翔. 小分子醇对废旧PU硬泡回收再利用的影响研究[J]. 中国塑料, 2019, 33(10): 84-88. |
| [10] | 耿浩然 章诚 夏艳平 徐蕙 马文中 陶国良. 力化学脱硫胶粉增韧聚丙烯的性能研究[J]. 中国塑料, 2017, 31(11): 41-47. |
| [11] | 黄继明 刘润清 韦恩光 饶红勇 吴思展. PET在离子液体[Pmim]OH催化下的液化行为研究[J]. 中国塑料, 2017, 31(11): 102-107. |
| [12] | 马立群, 董少波, 石佳, 王凤超, 王雅珍. 利用废旧聚丙烯腈纤维织物制备聚丙烯用抗老化剂[J]. 中国塑料, 2017, 31(03): 82-89 . |
| [13] | 姜秀娟 石锐 余成科 陈大福 张立群. 医用HMDI基聚碳酸酯型聚氨酯弹性体的合成与表征[J]. 中国塑料, 2012, 26(09): 22-27 . |
| [14] | 郭欣欣 张向京 刘玉敏 赵飒 胡永琪. 气固相法生产氯化聚氯乙烯树脂的研究进展[J]. 中国塑料, 2010, 24(08): 13-16 . |
| [15] | 封悦霞, 殷宁, 赵雨花, 李其峰, 王军威, 亢茂青, 王心葵. 聚碳酸酯二元醇的合成及聚氨酯弹性体的性能研究[J]. 中国塑料, 2007, 21(4): 20-24. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802034965号
京ICP备13020181号-2
京公网安备11010802034965号
京ICP备13020181号-2